Introduction: Acute lymphoblastic leukemia (ALL), the most common pediatric malignancy, is caused by the accumulation of multiple genomic aberrations in developing lymphocytes. In children, high hyperdiploidy (HeH) is the most prevalent subtype of B-cell ALL (B-ALL), which is associated with a superior outcome. However, because of the high incidence of HeH B-ALL in children, this subtype is responsible for about 25% of all relapses. The prediction of relapse remains difficult, especially in these good risk subtypes. Recent studies indicate that ALL is a heterogeneous disease, with multiple leukemia clones at diagnosis, each having a distinct set of mutations. We hypothesize that these different subclones have a different sensitivity to treatment and that samples with more heterogeneity are more prone to relapse. We used single-cell sequencing to determine the clonal heterogeneity of HeH B-ALL at diagnosis and followed the clonal evolution during chemotherapy treatment.
Methods: Bone marrow samples of 11 patients with HeH B-ALL were collected at diagnosis, during treatment (for 9 cases) and at relapse (for 1 case) (Table 1). Samples during treatment were enriched for leukemia cells using magnetic antibodies targeting cell membrane markers (CD10, CD19, CD34) with the MARS® gentle sorter. We performed targeted single-cell DNA sequencing with the Mission Bio Tapestri® Platform, using our custom amplicon panel of 414 amplicons across 39 commonly mutated genes. This method detected single nucleotide variants (SNVs) and small insertions/deletions (indels) based on targeted PCR amplification in about 4000 cells per sample.
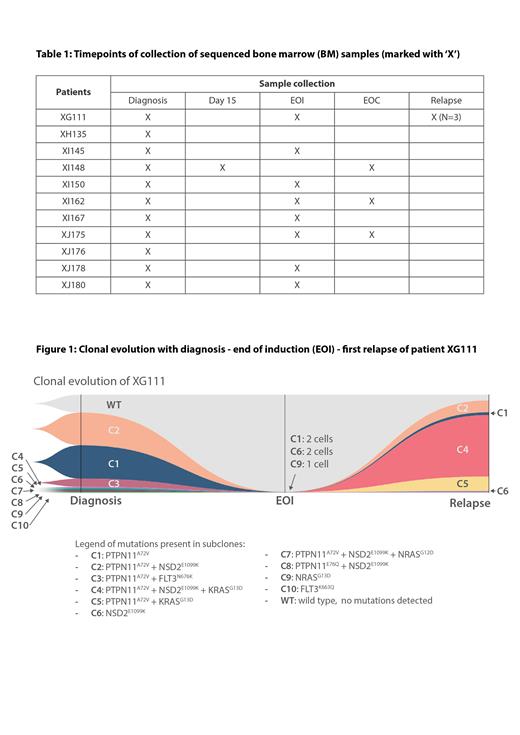
Results: We sequenced diagnostic samples from 11 HeH B-ALL patients. For 9 patients we also tested samples during treatment, either at day 15 of treatment (1 case) or at the end of induction (EOI) (8 cases), and for 3 cases an additional sample at the end of consolidation (EOC). Furthermore, for one patient, we analyzed samples of 3 subsequent relapses. In total, 117,148 single cells were processed, with an average of 4500 cells per sample. We detected multiple subclones in almost all diagnostic samples (9/11), ranging from 2 to 10 subclones per case. Most frequently mutated genes were KRAS, NRAS, PTPN11 and FLT3, as well as NSD2. Four cases had 2 or more different KRAS and/or NRAS subclones. Three cases had a subclone with a KRAS/ NRAS mutation co-occurring with a NSD2 mutation. For the patients with multiple subclones at diagnosis, we subsequently performed single-cell analysis on one or more follow-up samples, either during treatment or at relapse. In the samples taken at EOI, we detected residual leukemia cells in all 8 cases: multiple subclones were detected in 5 cases and only one subclone persisted in 3 cases. Residual leukemia cells were also detected in all samples collected at EOC, though in a lesser amount as compared to the previous timepoint sequenced. Interestingly, in the matched diagnosis-relapse case, we found that a minor subclone with a NSD2, PTPN11and KRAS mutation became the dominant clone at first relapse (Figure 1).
Conclusions: Single-cell DNA sequencing reveals a strong genetic heterogeneity in HeH B-ALL based on a variety of mutations in KRAS, NRAS, PTPN11, FLT3 and NSD2. RAS mutations and FLT3 mutations are mutually exclusive in subclones, whereas NSD2 mutations and RAS mutations are frequently co-occurring in the same subclone. This cooperation is highlighted in our relapse patient, having a minor subclone with a KRAS and NSD2 mutation at diagnosis that became the largest subclone at relapse. Even though few leukemia cells persisted during treatment, we were able to genetically identify the residual subclones with a potentially higher therapy resistance.
Disclosures
Cools:Janssen Pharmaceutica (Beerse, Belgium): Research Funding.